Articles & Studies

by John A. Maher, Juan P. Rodriguez and Scott A. Mischler
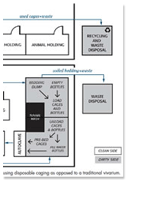
A study conducted by Pfizer Comparative Medicine and Charles River Laboratories sought to: a) Compare the ammonia removal efficiency of two ventilated cage systems in which the air injection and exhaust ports were either horizontal (CH) or vertical (CV), and b) Determine if using autoclaved bedding resulted in different intra cage ammonia concentrations than using non-autoclaved bedding.
Excerpt: "Intra-cage ammonia values were larger (P<0.01) for the CH than for the CV cages. All other things being equal, the moister, more nutrient rich environment in the CH cages may have favored a larger bacterial population, enhanced bacterial activity and thus greater NH3 levels."
SOURCE: Poster, AALAS National Meeting, Nov 2015.
by Mary Rainey, Ollie Dalisay, Eleanor Gonsalves, Evelyn Jaime, Roberto Leal, Rafael Soares, and Jerik Toloza
A study conducted by Bristol-Myers Squibb measured the ammonia buildup at 14 days between three different caging solutions.
Excerpt: "Dividing this ppm/hr exposure by the 24 hours the monitor was in the cage, the mice exposure to ammonia is less than 1 ppm/hour. We used the human OSHA Permissible Exposure Limit (PEL) - General Industry of 50 ppm/hr (8 hr TWA) as a reference... We are well below these reference points at the 14-day cage change timeframe."
SOURCE: Poster.
by Jason M Koontz, David M Kumsher, Richard Kelly III, and Jonathan D Stallings
A study conducted by the United States Army Medical Research Institute of Infectious Diseases uses adult Sprague-Dawley rats housed in the Innovive Disposable IVC Caging System. The study "sought to identify an optimal rodent bedding and cage-change interval to establish standard procedures for the IVC in our rodent vivarium."
Excerpt: "The 2-wk cage-change interval will not only markedly reduce labor time and material costs, especially when using disposable cages in an IVC system, but it will also allow studies that require prolonged exposures, treatments, or observations without disturbing the animals."
SOURCE: "Effect of 2 Bedding Materials on Ammonia Levels in Individually Ventilated Cages", by Jason M Koontz, David M Kumsher, Richard Kelly III, and Jonathan D Stallings. Published in JAALAS Vol 55, Jan 2016. Pages 25-28.
by Cliff Roberts, DVM, DACLAM
Results from a worldwide study on recyclable caging adoption and performance metrics should inform plans for facility designs, energy use, and operating efficiency. Cliff Roberts examines the use of recyclable caging technology for a variety of programs, facilities types, and census sizes in the University of California system and facilities worldwide.
He sets out selection criteria, and delivers aggregate data on facility square footage, quantity of cages, cage density per unit space, actual costs and estimated savings. He illustrates improved process flows, staffing levels, ergonomics, utility consumption, housing protocols, operating costs, biosecurity and waste handling for both new construction projects and renovations.
SOURCE: PowerPoint Presentation, Tradeline Animal Research Facilities Conference, Nov 2013.
by Rebecca Varrall, David Smith and Stephen Baker
Pfizer has selected the disposable cage as the primary Individually Ventilated Caging (IVC) option for a new 52,000ft2 research facility with a 7,500+ rodent housing capacity. This poster describes their experiences using disposable cages.
SOURCE: Poster, IAT Congress, April 2014.
by Rudy Cagneron, B.S.
Due to a lack of equipment required to house an incoming arrival of Nu/Nu+ mice inside the animal facility, XOMA (US) LLC conducted a study to analyze and compare the cleanliness of Innovive disposable caging to conventional static caging. The goal was to investigate if a disposable caging system would be a useful alternative compared to a standard caging system.
SOURCE: Poster Presentation, 62nd AALAS, Oct 2012.
by Dr. Richard Lin
Explora Biolabs needed to scale up their facility rapidly, and this article explains how disposable caging offered financial and operational benefits.
SOURCE: "Disposable Caging – How It Helped Our Lab Grow", by Dr. Richard Lin. Published in Animal Lab News, April 2010.
by Bret Tallent and Jaime White-James
Excerpt: "In August of 2013, the University of Arizona completed construction of a 19,000 square foot animal facility designed solely around the use of Innovive™ disposable, independently ventilated caging. At full capacity, the facility is designed to house over 3,500 cages of mice and 1,300 cages of rats. All of the cages, bedding, food and water are sterile, and the disposable caging and components are all recyclable with 70% of the material being reclaimed. This facility has no automatic washing equipment of any kind and only a single, small autoclave. As a result, we quickly discovered there is a unique learning curve to this type of facility operation."
by Hellen Kelly
A 22,000 mouse-cage facility, due to open later in 2013, will have no cage washing area due to their adoption of disposable caging technology. The use of disposable pre-filled water bottles enables them to save valuable time and money from bottle processing, filling, and autoclaving; avoid leakage or flooding; and prevent the risk of cross contamination.
SOURCE: “Fast Water”, ALN World Magazine, Jan/Feb 2013 issue.
by James Bell
The Human Genome Sciences’ new vivarium employed a new animal housing system that uses disposable caging. The new disposable caging system enabled HGS to decrease cost, labor and time, maximize space efficiency while simultaneously providing a safe and healthy environment for research animals and scientists. Vivaria built with disposable caging systems can cost two-thirds less to build compared to the ones with traditional housing systems. HGS’s experience matched this cost savings and also saw that disposable caging reduces per diem cost.
SOURCE: Poster for Oct. 2012
by Jim Fallon
Disposable caging has been rapidly adopted around the world at facilities of all sizes as an improvement over washing and sterilizing cages on site. Driving this adoption is the desire by institutions to simplify operations, standardize cage change protocols and reduce the burden on animal care staff.
SOURCE: "Disposable Caging: Simplify Processes and Reduce Biosecurity Risk at Your Facility", by Jim Fallon. Published in ALN, September, 2011
by Jerald Silverman
Third-party research indicates that Innovive disposable cages have 15x lower levels of NH3 than traditional caging, and also features a gentle animal environment.
Excerpt: "This study compares reusable and disposable individually ventilated mouse cages in terms of the formation of intracage CO2 and NH3. Nevertheless, over the course of the study, we found significantly higher NH3 concentrations in the reusable IVCs."
SOURCE: "Ammonia and Carbon Dioxide Concentrations in Disposable and Reusable Ventilated Mouse Cages", by Jerald Silverman, David W Bays, Sheldon F Copper, and Stephen P Baker. Published in JAALAS Vol 47, March 2008.
This study was performed to evaluate daily intracage ammonia (NH3) levels produced by mice housed in the Innorack3 3.0 IVC system.
Excerpt: "The Innorack® 3.0 ventilation system employs transversal airflow that provides efficient evacuation of moisture and gases from the Innocage®, resulting in a cleaner environment for the mice and more time in between cage change-outs."
by Doug Ferguson, University of Calgary
In a study published in the Proceedings of the National Academy of Sciences (PNAS), researchers in Deborah Kurrasch’s lab at the University of Calgary have provided evidence that BPA and BPS cause alterations in brain development leading to hyperactivity in zebrafish.
Excerpt: "In the second trimester, brain cells become the specialized neurons that make up our brain. What we show is that the zebrafish exposed to BPA or BPS were getting twice as many neurons born too soon and about half as many neurons born later, so that will lead to problems in how the neurons connect and form circuits."
SOURCE: "Bisphenols Affect Zebrafish Embryonic Brain Development", by Doug Ferguson, University of Calgary. Published in ALN, January 2015.
by Dr. Marek Piechowiak
In September 2007, the animal facility of a Biomedical Research Laboratory needed a significant renovation. Before undertaking any major investment decisions, the company decided to evaluate the Innovive Disposable Rodent Caging System and, specifically, its ability to support the company's animal breeding program.
SOURCE: "Evaluation of Disposable Caging System as a Breeding and Husbandry Solution", as presented at the 2008 AALAS National Meeting.
The Innovive Disposable Rodent Caging system has been shown to maintain low ammonia levels, improve transgenic breeding and facilitate the prevention/eradication of disease.This paper gives an in-depth, technical description of the Innovive airflow system.
SOURCE: "Assessment of Ventilated Rodent Cage Air Exchange Using an Optical Detection Method", by Innovive, 2012.
The Guide for the Care and Use of Laboratory Animals states that sanitization of caging accessories (for example, filter tops and wire-bar lids) should be done every 2 weeks. This study examines how frequent a cage change should ideally occur.
SOURCE: "Investigation of Appropriate Sanitization Frequency for Rodent Caging Accessories: Evidence Supporting Less-frequent Cleaning", by Curtis W Schondelmeyer, Dirck L Dillehay, Sonji K Webb, Michael J Huerkamp, Deborah M Mook, and Jennifer K Pullium. Published in JAALAS Vol 45, No 6, Nov 2008.
by Marie-France Champy, Isabelle Goncalves Da Cruz, Elodie Bedu, Roy Combe, Benoit Petit-Demoulière, Stéphanie Muller, Abdelkader Ayadi, Yann Herault, & Hamid Meziane
Consistent phenotyping data within and across labs require standardization of protocols but also of housing conditions, which is well known to influence mouse phenotype characteristics such as behavior. The present study was designed to evaluate the impact of 3 different IVC systems on mouse phenotypes.
SOURCE: Poster Presentation, 62nd AALAS, Oct 2012.
by American Chemistry Council (ACC)
Press release excerpt: "Based on the LCI study results and data from U.S. EPA, the generation of cleaned recycled resin required 71 trillion Btu less than the amount of energy that would be required to produce the equivalent tonnage of virgin PET and HDPE resin. The corresponding savings in greenhouse gas emissions was 2.1 million tons of CO2 equivalents, an amount comparable to taking 360,000 cars off the road."
SOURCE: "New Study Confirms Recycling Plastic Significantly reduces energy use and Greenhouse Gas Emissions" - press-release (April 28, 2010)
by Danielle Bornstein-Elbirt DVM MS DACLAM, Lauren Kelley BS RLATG, Jessica Cabral BS RLATG, Stephen Baker MS CMAR
Comparative Medicine evaluated Innorichment™ to assess the quality of nests in comparison to other paper products already in use in the facility. In addition, CM wanted to determine if any efficiency could be achieved by using a pre-bedded, pre-enriched cage compared to having to manually add the enrichment at the time of cage change. Using a scoring system published by Hess et al, it was determined that mice enriched with Innorichment™ built nests with an median score of 3.75 and on average it took technicians 3 seconds less per cage using this type of enrichment.
SOURCE: "Evaluating a New Environmental Enrichment Option For Mice", by Danielle Bornstein-Elbirt, Lauren Kelley, Jessica Cabral, Stephen Baker. Published in 2015.
by Lacey A. Mock, RLAT, Kathleen M. McDonald, B.S., CMAR, RLATG, and Joseph T. Newsome, DVM, DACLAM
This article highlights a few critical factors to consider when selecting specific enrichment hut products, such as construction materials, the true enrichment value, the duration of use, benefits to the animal, and cost. The three options that were compared are cardboard huts, reusable plastic huts, and single-use recyclable plastic huts.
Excerpt: "Most importantly, the use of single-use, recyclable huts and wheels notably improved animal welfare. Reduction of stereotyped food chewing behavior reduced cage handling stress as well as incidences of blocked sippers and associated cage flooding. Similarly, reduction in barbering reduced animal stress, injury, and associated clinical interventions. Another positive outcome from our study we noted, the mice remained engaged with huts and running wheels throughout the duration of placement, making them a valuable and beneficial enrichment tool."
SOURCE: "Enrichment Cost Considerations and Effects on Stereotyped Behaviors in Mice", by Lacey A. Mock, Kathleen M. McDonald, and Joseph T. Newsome. Published in ALN, April 2016.
by Bret R. Tallent, Latg, and Jonathan Lifshitz, PhD.
Antagonistic behavior in group-housed rodents is a reoccurring issue that can not only affect the welfare of the animals but also impact the validity of research results. Find out how a team of researchers were able to reduce aggressive behavior in male mice by upwards of 65% by using custom built cage dividers.
Excerpt: "Mice reared in a complex cage system that emulates a burrow-like environment will lead to healthier, less reactive mice. Few publications evaluate the effects of cage dividers or complex caging in mic. Of those that do, the modifications include a much larger cage, or caging system, that varied in the complexity of customization to cages. No simple, cost effective home-cage interventions are available to reduce aggression and maintain animal welfare. Therefore, we conducted an observational study of group-house mice within standard disposable individually ventilated mouse cages with the addition of custom built dividers."
SOURCE: "Reducing Aggressive Behavior in Mice with the Addition of Cage Dividers", by Bret R. Tallent, Latg, and Jonathan Lifshitz, PhD. Presented at 2017 AALAS National Meeting in Charlotte, NC.